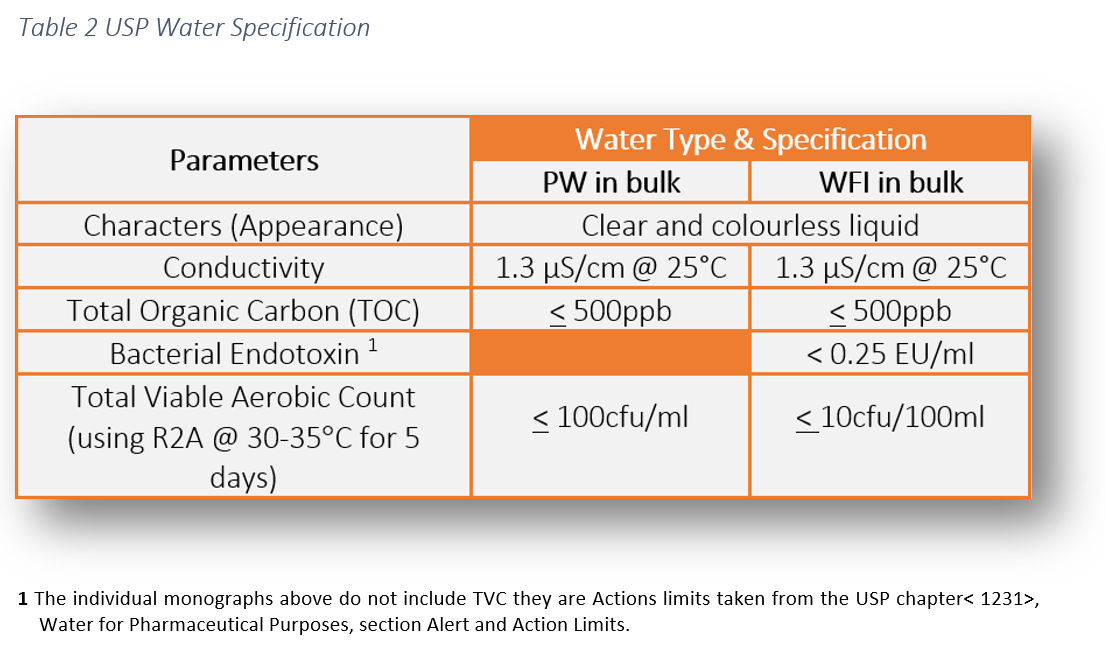
Action levels in usp 1231 100cfu ml for purified water and 10cfu 100ml for water for injection are generally considered to represent a level above which the water is unfit for use.
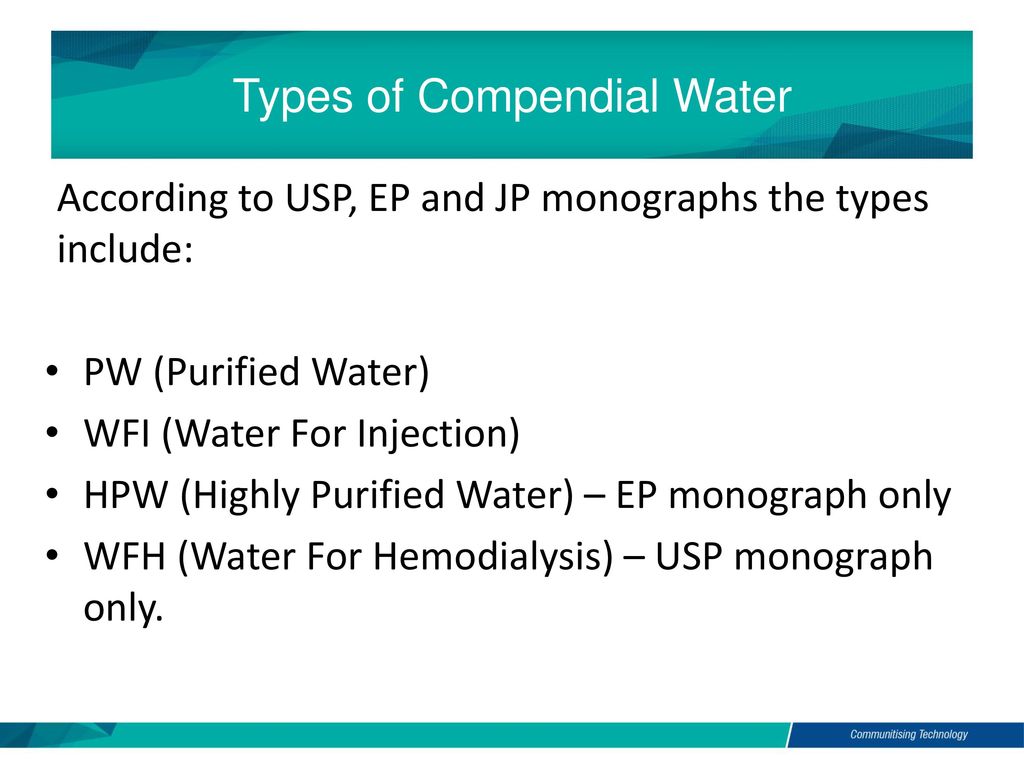
Usp monograph for purified water.
The procedure described below is designed for measuring the conductivity of purified water and water for injection.
You may purchase usp24 by calling customer service at 800 877 6733.
Purified water is water obtained by a suitable process.
Water under 8 terms and definitions in the plastic containers of not larger than 1 l size.
Including total vial count and.
For sterile purified water in containers having a fill volume of less than 50 ml add 0 4 ml of 0 1 n potassium permanganate and boil for 5 minutes.
Usp sterile water for irrigation the usp designation means that the water is the subject of an official monograph in the current us pharmacopeia with various specifications for each type.
Glass containersgeneral notices and requirements where used for sterile dos.
We can not provide photocopies of copyrighted material.
It is prepared from water complying with the u s.
Purified water purified water see usp monograph is used as an excipient in the production of nonparenteral preparations and in other pharmaceutical applications such as cleaning of certain equipment and nonparenteral product contact components.
Preserve in single dose glass or fied see 8 230.
Environmental protection agency national primary drinking water regulations or with the drinking water usp29 regulations of the european union japan or with the world health organization s guidelines for drinking water quality.
Stage 1 of the procedure below may alternatively be performed with the appropriate modifications to step 1 using on line instrumentation that has been appropriately calibrated whose cell constants have been accurately determined and whose temperature compensation function has.
It contains no added substance.
That is why an oos investigation must be undertaken if those action levels are exceeded.
5220 1231 water for pharmaceutical purposes general information first supplement to usp 35 nf 30 dbp levels in drinking water can be minimized by using purified water purified water see the usp monograph disinfectants such as ozone chloramines or chlorine diox is used as an excipient in the production of nonparenteral ide.
Usp24 contains complete versions of all pharmaceutical water monographs p.
1752 1754 and the general chapters 643 toc 645 water conductivity p.
1927 1929 and 1231 water for pharmaceutical purposes p.